Related Vendor
Stabilizing plasmids
A number of different technologies now exist for conducting ALE experiments. In addition to the mode of operation, the scale of the bioreactors used is a primary differentiating factor. One such technique is cultivation of microorganisms in a continuous process at constant flow rate in a lab bioreactor with a limited supply of nutrient medium and outflow of cells and (by)products over an extended period. Adaptation and diversification of the cells can take place depending on the chosen selection pressure, e.g. by slowly increasing the concentration of a specific media component at the bioreactor supply.
The stability of plasmids introduced is a fundamental challenge with continuous processing using genetically modified production strains under limiting growth conditions [12], because any plasmid loss results directly in the loss of the production path and consequently to superior fitness of plasmid-free cells. Alternatively, an extension of the conventional batch process for lab evolution can be used. Individual cultivation batches are sequentially inoculated until the desired number of generation cycles is reached. Normally a surplus amount of all growth-relevant media components is supplied to minimize the risk of plasmid loss.
Miniaturized and automated
The manual effort involved in ongoing inoculation and monitoring of biomass and product formation is a disadvantage when the batch is processed in conventional shaking flasks. To address this problem, an intensive effort is being made to develop improved ALE techniques. A number of different approaches are being evaluated which make use of miniaturization and automation.
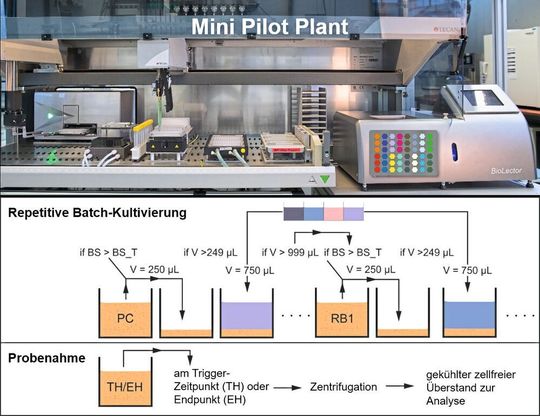
In the Bioprocesses and Bioanalytics working group at the Institute for Bio- and Geosciences at Forschungszentrum Jülich, additional automated workflows for undirected strain optimization using ALE were added to an existing cultivation platform which was already highly automated (Mini Pilot Plant) [13]. Fully automated repetitive batch cultivation (Fig. 2) can be performed using a modularized platform which combines among other things a microtiter plate based micro-bioreactor system (e.g. Biolector, m2p-labs) and a liquid handling system (e.g. Freedom Evo, Tecan). Preparation of different media compositions, cool temporary storage of the media and inoculation of the individual cavities in the cultivation plate in the micro-bioreactors take place directly on the robotic deck using liquid handling operations.
Subsequent inoculation of the grown bacteria cultures in fresh medium (next batch cycle) is also automated using a time trigger or online signals (e.g. backscatter measurement) available from the integrated cultivation unit. This makes it possible to ensure for example that the cultures are exactly in the exponential growth phase at the time of transfer. This is another advantage compared to conventional manual ALE processing in shaking flasks or bioreactors where directly usable online signals are not available.
Tested in academic environment – heading for industrial applications
![Fig.3: Automated lab evolution with C. glutamicum to increase growth on D-xylose as the sole carbon and energy source (top). When a 48-well microtiter plate is used, in the selected inoculation schema four independent cultivation replicates can be produced and samples taken twice during the process to analyze the (by) product spectrum. Modified by [13] Fig.3: Automated lab evolution with C. glutamicum to increase growth on D-xylose as the sole carbon and energy source (top). When a 48-well microtiter plate is used, in the selected inoculation schema four independent cultivation replicates can be produced and samples taken twice during the process to analyze the (by) product spectrum. Modified by [13]](https://cdn1.vogel.de/unsafe/540x0/smart/images.vogel.de/vogelonline/bdb/1435300/1435328/original.jpg)
With the new ALE techniques, the assimilation properties of a previously constructed C. glutamicum strain for the C5 carbohydrate D-xylose were significantly improved (Fig. 3) [11,13]. This approach also made it possible to reliably test the resilience and long-term stability of new strain constructs under process-relevant conditions [4].
So far, this high-performance lab evolution platform has been developed and used in an academic environment. At the Microbial Bioprocess Lab (Mibiolab), further development work, which has clear industrial relevance, and successful deployment of these technologies will take place in collaboration with industrial partners. Mibiolab follows the Open Innovation Labs approach, meaning that it is an enabling space for developing new solutions to address relevant industrial challenges and issues. Mibiolab offers a broad spectrum of cooperation options and partnerships including R&D service contracts for industrial and academic partners.
(ID:45277008)





:quality(80)/p7i.vogel.de/wcms/06/a1/06a11f1d752afb35854aa9e1afb62e98/0129209625v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/6d/df/6ddf023361ec7f8fbfb6c56521577960/0129056554v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/26/3d/263d97f8f2797267ecf2781b56882758/0129056451v1.jpeg)
:quality(80)/p7i.vogel.de/wcms/63/0c/630cf9ab0cafdcdd9b33802d53c5b943/0128938770v1.jpeg)
:quality(80)/p7i.vogel.de/wcms/21/03/21030cc2541c455da0769decca8d1f82/0129146048v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/bd/aa/bdaae5b9d381e2471ab37f479ad43d79/0129143884v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/8f/66/8f66b8acbb3037ca8e6b320bcad1c817/0129118043v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/66/82/66825163ab842baded640db1a484e729/0129117548v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/90/f8/90f8c3152a59205a7cac1cc182eb186b/0129209634v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/53/ac/53acd3796c0d43ad0f7cfd80dd7ab82e/0129209615v1.jpeg)
:quality(80)/p7i.vogel.de/wcms/a3/cd/a3cdc440f6f0f426b85042082678018f/0129165252v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/6c/15/6c15f7685b6076b906f2d572f6c71bb8/0129103298v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/d1/3c/d13c2ad34af600dbd360c0bb0990b922/0129045715v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/e1/b3/e1b3e26d03101ba93b76b304da150c9a/0129025388v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/75/db/75db8afe45607bee750df705e22b60e9/0128938816v1.jpeg)
:quality(80)/p7i.vogel.de/wcms/85/42/85423f3382c25d1ae0ac5645e969cd36/0128898289v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/f1/ea/f1eac0379480f4aa25ab2db9e0a396ed/0129209654v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/34/a4/34a44fab2ba6af4b5f7cb8c3ecfc6362/0129207768v1.jpeg)
:quality(80)/p7i.vogel.de/wcms/a4/12/a412474313fc7d83bc51a4b99ef0af73/0129189645v2.jpeg)
:quality(80)/images.vogel.de/vogelonline/bdb/1710600/1710675/original.jpg)
:quality(80)/images.vogel.de/vogelonline/bdb/1682500/1682579/original.jpg)
:quality(80)/images.vogel.de/vogelonline/bdb/1677000/1677080/original.jpg)
:quality(80)/p7i.vogel.de/wcms/1b/a4/1ba4d882cb5a04d10b40f9a9bd7912d8/0122923567v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/85/42/85423f3382c25d1ae0ac5645e969cd36/0128898289v2.jpeg)